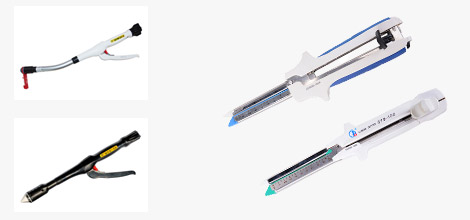
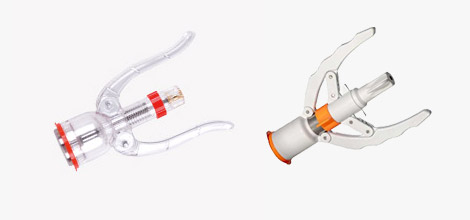
The Need for Proper Use of Sugical Staplings
If you've been following medical device safety issues, chances are you've seen the news of patient injuries and deaths associated with Sugical Staplings. These devices are commonly used in many high-risk surgical procedures. Misuse and malfunction of Sugical Staplings can lead to serious complications.

The first step is to recognize how complications can occur by understanding how Sugical Staplings work.
Sugical Staplings are available in different designs for different applications. For example, linear anastomoses are used to seal and transect (staple and cut) internal tissue during endoscopic surgical procedures such as organ removal, as well as bariatric and other gastric procedures. Round models are most commonly used for gastrointestinal anastomoses. The linear design consists of a handle and shaft with jaws on the end that can be closed over the tissue of interest (think of an alligator jaw). The surgeon clamps the tissue and fires the Sugical Stapling. This operation deploys rows of staples into the clamped tissue on either side of the intended cut line, and then immediately uses a knife to transect the tissue between these staple lines. In this manner, the Sugical Stapling seals the tissue on either side of the incision to prevent bleeding. In contrast, the circular model includes a circular anvil holder that is compressed at the end of the cartridge to seal and cut the tissue of interest.
However, bleeding or gastrointestinal leakage may occur if the Sugical Stapling staple line is incomplete or cannot be secured. These problems may become apparent immediately or may not appear until some time later, resulting in severe symptoms long after the patient is closed and sent to recovery. In these events, the patient's injuries can range from no harm to death. We have found that in most cases, Sugical Staplings actually function as expected. Conversely, clamping another instrument or clamp, holding tissue that is too thick or too thin, or other forms of misuse can lead to adverse outcomes. Poor staple line integrity can also occur with lesions, necrotic or ischemic tissue.
The following are key recommendations that we recommend hospitals and providers take immediately.
(1) Improve event reporting. A common complaint about the FDA's MAUDE and ASR databases is that they provide incomplete or inaccurate information. Risk managers and patient safety professionals can make attempts to improve the quality of incident reporting.
(2) Consider users when making purchasing decisions. Supply chain and purchasing departments should consider user familiarity when making major purchasing decisions. User unfamiliarity continues to be a contributing factor to investigations and problem reporting. Involve users in purchasing decisions to reveal potential usage problems before the equipment is put into service.
(3) Identify risks and plan ahead. Users of these Sugical Staplings should be aware of the risk of immediate and severe bleeding in vascular applications. We encourage users to plan for back-up interventions in the event of bleeding, an approach that can greatly reduce patient harm.
(4) Improve user training. Ensure that surgeons have knowledge and experience with the device. The availability of Sugical Staplings may vary by model. Unexpected outcomes are less likely to occur if the surgeon has knowledge and experience with the device prior to use. Work with the manufacturer to develop appropriate Sugical Stapling training standards and ensure that these standards are met prior to using the Sugical Stapling.
This is all about the need for proper use of Sugical Staplings. If you need more detailed information, please feel free to contact us!



 info@qjmed.com
info@qjmed.com